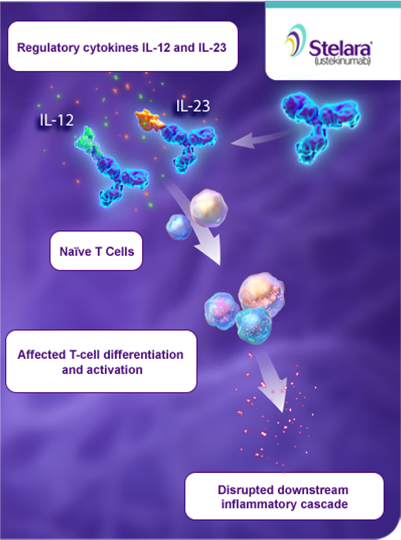
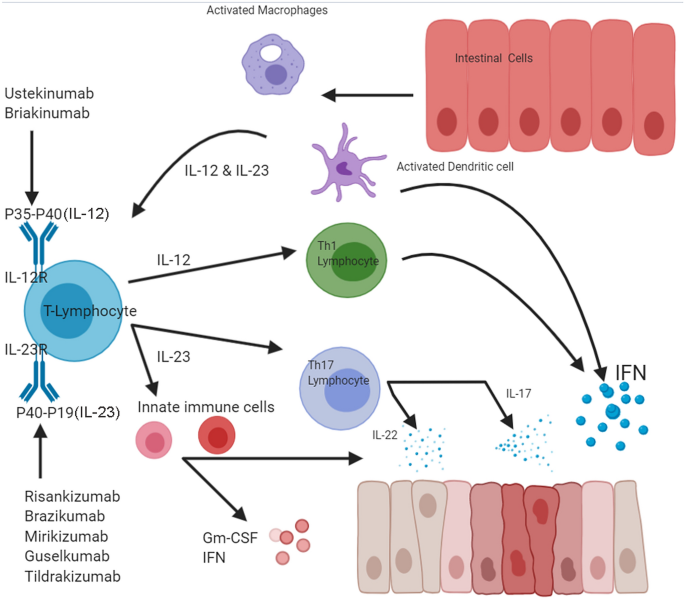
Ustekinumab (Stelara, Janssen), which was recently approved by the US Food and Drug Administration (FDA) for the treatment of moderate to severe Crohn's disease, is an antibody directed against the p40 subunit shared by IL12 and 23May 22, 19First to market was Johnson &Consistently, neutralizing antibodies against IL12/IL23 p40 and IL23 p19 have been successfully used in clinical trials for therapy of Crohn´s disease and pilot studies in
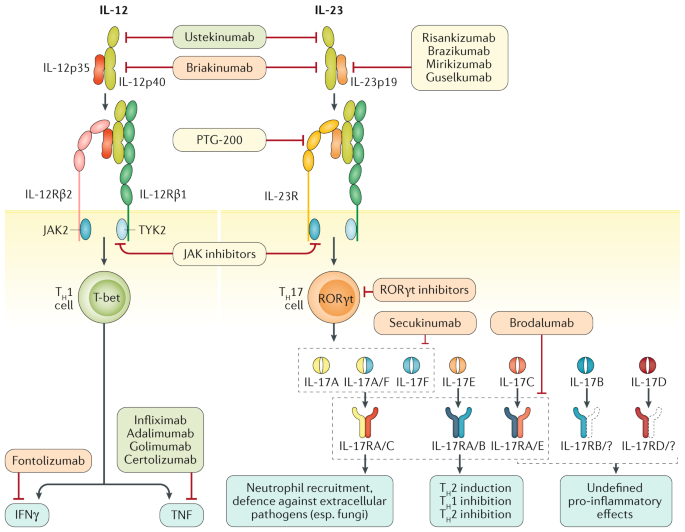
Il 12 Il 23 And Il 17 In Ibd Immunobiology And Therapeutic Targeting Nature Reviews Gastroenterology Hepatology
Il-23 inhibitor crohn's
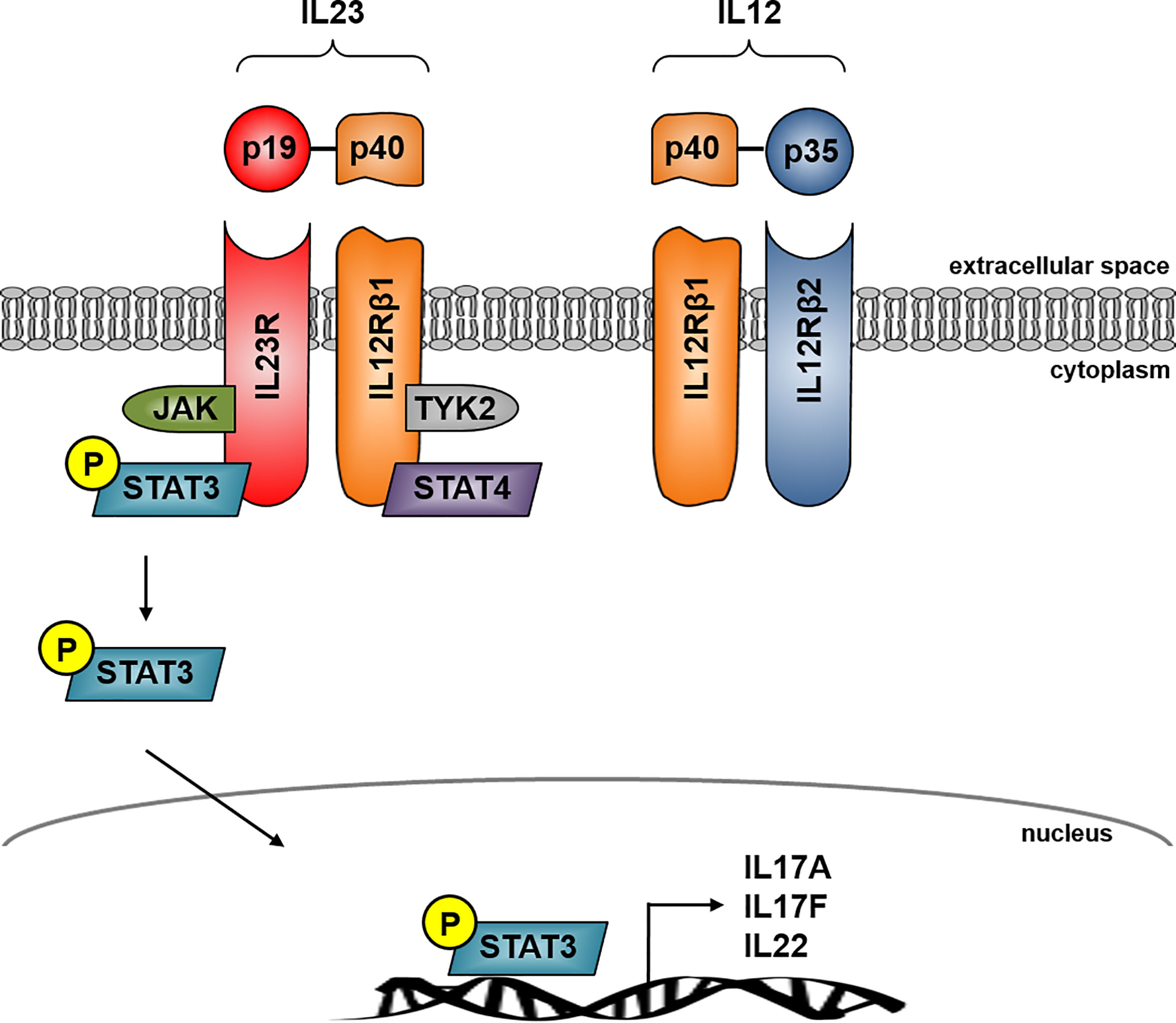
Il-23 inhibitor crohn's-Risankizumab, guselkumab, and tildrakizumab are new IL23 inhibitors currently in phase 3 trials with promising early efficacy and safety resultsFunction The protein encoded by this gene is a subunit of the receptor for IL23This protein pairs with the receptor molecule IL12Rβ1 (), together forming the IL23 receptor complex, and both are required for IL23 signalingThis protein associates constitutively with Janus kinase 2 (), and also binds to transcription activator STAT3 in a liganddependent manner




Il 23 In Inflammatory Bowel Diseases And Colon Cancer Sciencedirect
Nov 26, 19Three IL17 antagonists, secukinumab, ixekizumab, and brodalumab have been approved for the treatment of psoriasis, with additional IL17 inhibitors under development In addition, ustekinumab is a combined IL12 and IL23 blocker approved for the treatment of moderatetosevere plaque psoriasis (MTSPP), psoriatic arthritis, and Crohn's diseaseFeb 01, 19The interleukin (IL)12 family of cytokines, including IL12 and IL 23, play an important role in driving aberrant Th1 and Th17 immune responses in patients with Crohn's disease (CD) Targeting this pathway has opened new avenues for therapeutic interventionSep 17, 19The IL23 inhibitors guselkumab, tildrakizumab, and risankizumab selectively target the p19 subunit and inhibit IL23, but not IL12 IL12 is now thought to have antiinflammatory properties in psoriasis, playing a role in defence against intracellular pathogens, and inhibiting IL23 alone has been shown to be preferable to inhibiting IL12/IL
May 24, 16B Feagan et al Efficacy and safety of induction therapy with the selective IL23 inhibitor risankizumab (BI ), in patients with moderatetosevere Crohn's disease Results of a randomizedJul 14, "Tremfya is the first and only selective IL23 inhibitor approved for both active psoriatic arthritis and moderate to severe plaque psoriasis, asMar 09, The only IL23 inhibitor commercially available in the rheumatology space is Tremfya (guselkumab, Janssen), Mahadevan said "If all goes well, we'll have several IL23 inhibitors to work with for
May 25, 16Risankizumab (being codeveloped by Boehringer Ingelheim and AbbVie) is a monoclonal antibody against IL23p19 subunit 1 and has been given to patients with active severe Crohn's disease, many of whom had been previously treated with TNF inhibitors There were few severe or serious adverse events in risankizumabMay 03, 19IL‐23 is a heterodimer composed of two subunits p40, which is shared with IL‐12, and p19 3 Data from long‐term clinical trials and a large safety registry (Psoriasis Longitudinal Assessment and Registry;Oct 07, 18Allergan has commenced two clinical research programmes, INTREPID and EXPEDITION, to assess the safety and efficacy of its investigational IL23 inhibitor therapy called brazikumab for inflammatory bowel disease (IBD) The multicentre, randomised, doubleblind, doubledummy, placebocontrolled and activecontrolled, parallelgroup Phase IIb




Biological Agents Evaluated In Inflammatory Bowel Disease Download Table




Mechanisms Of Molecular Resistance And Predictors Of Response To Biological Therapy In Inflammatory Bowel Disease The Lancet Gastroenterology Hepatology
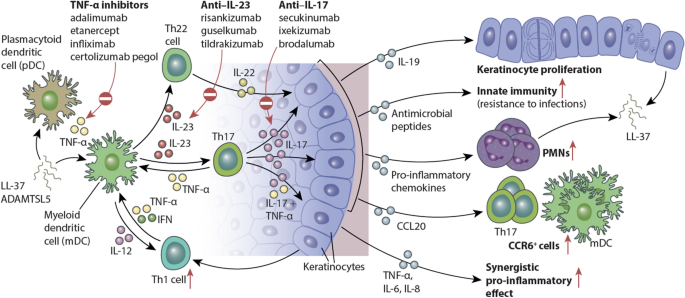
IL23 inhibitors have shown to be effective to both psoriasis and CD Crohn s disease, IL17 inhibitor, IL23 inhibitor Background Psoriasis is a chronic, recurrent, inflammatory skin disApr 29, IL23 has emerged as an important inflammatory cytokine in the pathogenesis of psoriasis We continue our series, Therapeutic Cheat Sheet, with a closer look at IL23 inhibitors Overall, IL23 inhibitors have demonstrated superior efficacy and safety in the treatment of psoriasis The relatively infrequent dosing is ideal with regard toJun 02, 21SKYRIZI is an interleukin23 (IL23) inhibitor that selectively blocks IL23 by binding to its p19 subunit 15,16 IL23, a cytokine involved in inflammatory processes, is thought to be linked to a number of chronic immunemediated diseases, including Crohn's disease 15 In April 19, SKYRIZI received US Food and Drug Administration approval




A Role For Il 12 In Ibd After All Sciencedirect



Inflammatory Bowel Disease Arthritis Rheumatism
Likewise, in a study of an IL23 inhibitor in Crohn's disease, patients with higher baseline serum concentrations of IL22, a cytokine induced by IL23, were more likely to respond More research is needed on biomarkers Piecing together various biomarkers is more likely to predict which is the best target or targets for a given patientMay 24, 16B Feagan et al Efficacy and safety of induction therapy with the selective IL23 inhibitor risankizumab (BI ), in patients with moderatetosevere Crohn's disease Results of a randomizedMar 01, 18The use of IL12/IL23 inhibitors, such as ustekinumab, is now also possible in Crohn's disease (CD), providing another example of the successful translation of immunological targeting into clinical practice Receive our free quarterly newsletters and your choice of journal publication alerts, straight to your inbox




Classes Of Biologics For The Treatment Of Ibd Alpco
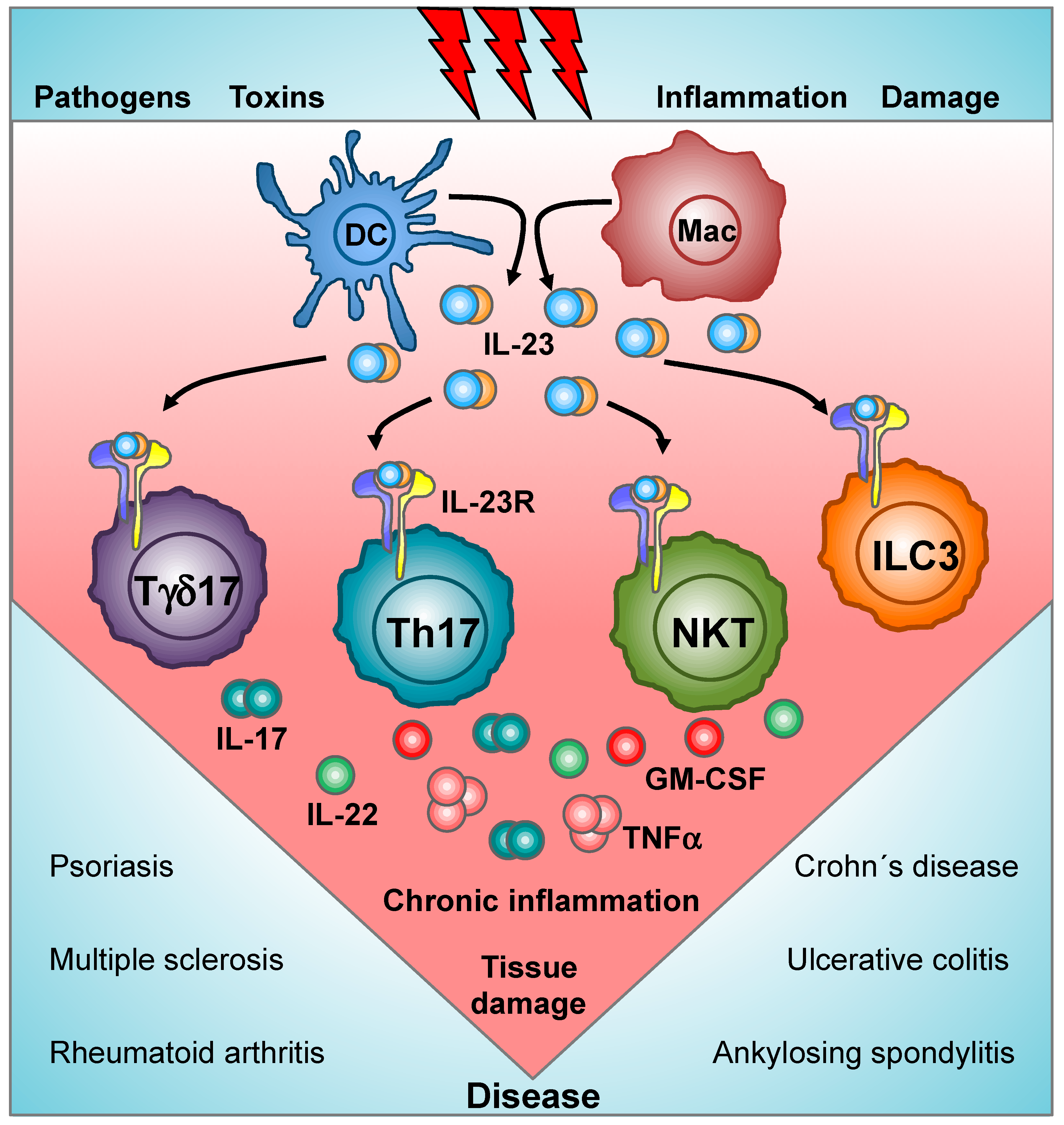



Cells Free Full Text Decoding Il 23 Signaling Cascade For New Therapeutic Opportunities Html
These biological agents include ustekinumab the first agent of this pharmacological class which has shown clinical efficacy in psoriasis, psoriatic arthritis, and moderatetosevere Crohn's disease (CD) and other monoclonal antibodies under investigation, including brazikumab, risankizumab, mirikizumab, and guselkumabThe IL12/IL23 axis is one of many proposed mechanistic pathways of intestinal inflammation 4 For years, IL12 was advocated as a key cytokine in IBD pathogenesis 5 However, with the discovery of IL23, subsequent studies revealed that IL12 inhibitors, which resulted in amelioration of inflammation in animal models, provided this effectSTA5326 is the first and only oral, smallmolecule, selective inhibitor of the interleukin (IL)12 cytokine family, including IL12 and IL23 These cytokines play a central role in inflammatory diseases such as Crohn's disease and other chronic inflammatory diseases




Il 23 In Inflammatory Bowel Diseases And Colon Cancer Sciencedirect
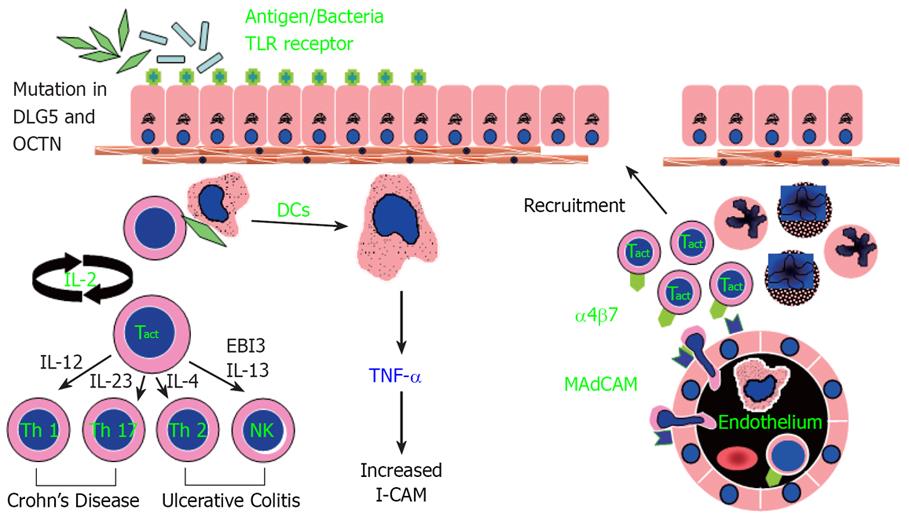



Immunopathogenesis Of Inflammatory Bowel Disease
Aug 01, 19In Crohn's disease, IL23 inhibition with p40 (ustekinumab) and p19 (risankizumab) inhibitors has demonstrated efficacy in phase II/III studies15 16 In contrast, a phase II study of IL17A inhibition with secukinumab did not meet its primary outcome and a phase II study of brodalumab, an IL17RA inhibitor, was prematurely stopped, withNov 30, 16AbbVie Receives Orphan Drug Designation for Investigational IL23 Inhibitor Risankizumab from the US Food and Drug Administration for the Treatment of Pediatric Patients with Crohn's Disease Orphan Drug Designation program provides orphan status to medications intended for the safe and effective treatment, diagnosis or prevention of rareMay 26, 19Currently, there are three classes of biologicals available to treat IBD These are antagonists to tumor necrosis factor (TNF), antiintegrins and inhibitors of interleukin (IL) 12/IL23 Unfortunately, primary nonresponse is observed in –30% of patients, and another 30% of patients become refractory due to secondary loss of response




Spotlight On Risankizumab And Its Potential In The Treatment Of Plaque Ptt




Personalised Medicine In Crohn S Disease The Lancet Gastroenterology Hepatology
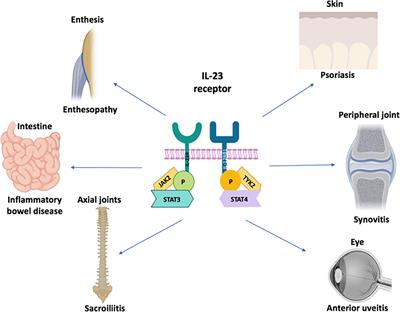
Mar 13, IL23 inhibitors are a type of biologic that can treat moderatetosevere psoriasis Learn more about these medications, including how they work, the possible side effects, and moreMoreover, genomewide association studies have revealed that variants of the gene encoding the IL23 receptor, as well as the locus harboring the gene encoding the p40 chain, confer genetic risk for developing Crohn's disease (CD) and ulcerative colitis (UC)Jan 21, 21IL17, IL23 Inhibitors May Outperform Older Biologics, Oral Therapies January , 21 Ilya Petrou, MD Ilya Petrou, MD Dermatology Times, Dermatology Times, February 21 (Vol 42, No 2), Volume 42, Issue 2 Several systemic therapies are currently available for the treatment of patients with moderatetosevere psoriasis




Il 23 In Inflammatory Bowel Diseases And Colon Cancer Sciencedirect




New Biologic Therapies That Target The Il 12 23 Pathway Youtube
Oct , 15d IL17 inhibition exacerbates Crohn's disease and Abcb1a / mouse colitis d IL17 inhibition weakens intestinal epithelial barrier function d IL23 inhibition attenuates Crohn's disease and Abcb1a / mouse colitis d IL23 inhibition promotes a regulatory, antiinflammatory environment in the gut Authors Joseph R Maxwell, Yu Zhang, WilliamApr 02, 21Risankizumab (SKYRIZI), an interleukin23 (IL23) inhibitor, is being evaluated as a treatment for adults with moderate to severe Crohn's disease and several other immunemediated conditions 1,2,7,8;Mar 23, 21The IL23 inhibitors include Stelara (ustekinumab), Tremfya (guselkumab), Ilumya (tildrakizumabasmn) and Skyrizi (risankizumabrzaa) while the IL17 inhibitors include Cosentyx (secukinumab), Taltz (ixekizumab) and Siliq (brodalumab)




Il 23 Inhibition In Psoriasis A Novel Approach To Convenient Consistent Clearance European Medical Journal




Induction Therapy With The Selective Interleukin 23 Inhibitor Risankizumab In Patients With Moderate To Severe Crohn S Disease A Randomised Double Blind Placebo Controlled Phase 2 Study The Lancet
Oct 08, 18numerical worsening of Crohn's disease in the treatment groups for both studies17 18 Therefore, while preclinical data suggested a role for both IL23 and IL17A in the pathogenesis of Crohn's disease, inhibition of these cytokines led to divergent results in clinical trials Indeed, it has been suggested subsequently that IL17AMay 24, 16B Feagan et al Efficacy and safety of induction therapy with the selective IL23 inhibitor risankizumab (BI ), in patients with moderatetosevere Crohn's disease Results of a randomized, doubleblind, placebocontrolled Phase II study Digestive Disease Week, San Diego, USA, 21–24th May 16 Abstract IDAug 07, 18Click here to read more about IL23 inhibitors At a July 18 public meeting of the New England Comparative Effectiveness Public Advisory Council, the group voted that, compared with anti—tumor necrosis factor (antiTNF) drugs, both guselkumab and risankizumab offered a superior benefit based on currently available data
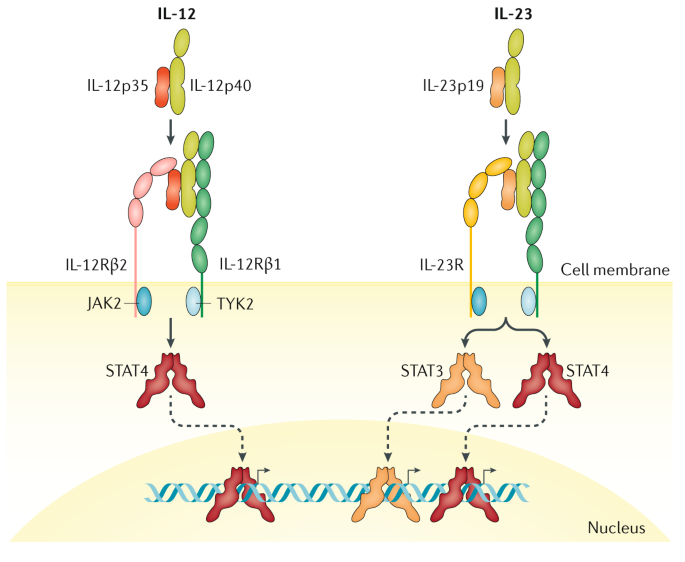



Il 12 Il 23 And Il 17 In Ibd Immunobiology And Therapeutic Targeting Nature Reviews Gastroenterology Hepatology




Targeting Interleukin 23 In The Treatment Of Noninfectious Uveitis Ophthalmology
Jan 07, 21SKYRIZI is an interleukin23 (IL23) inhibitor that selectively blocks IL23 by binding to its p19 subunit 15,16 IL23, a cytokine involved in inflammatory processes, is thought to be linked to a number of chronic immunemediated diseases, including Crohn's disease 15,16 In April 19, SKYRIZI received US Food and Drug Administration approval for the treatment ofOct 12, About a year and a half after presenting positive topline results in Crohn's disease, Eli Lilly gave its experimental IL23 antiinflammatory mirikizumabPSOLAR) have shown the IL‐12/23p40 inhibitor ustekinumab to be well tolerated in patients with psoriasis 2226 However, another




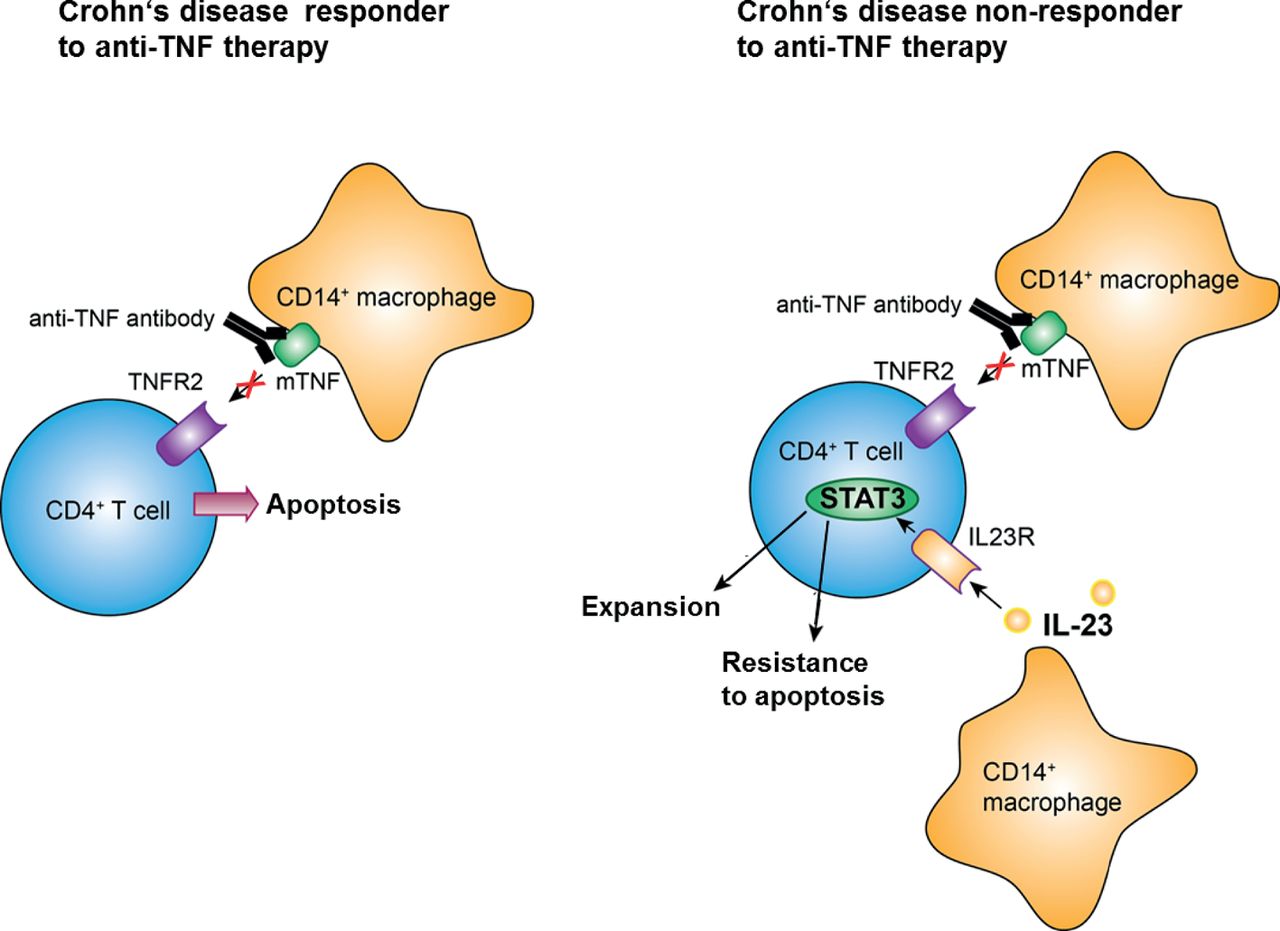
Expansion Of Il 23 Receptor Bearing Tnfr2 T Cells Is Associated With Molecular Resistance To Anti Tnf Therapy In Crohn S Disease Gut




Ibd Safety And Positioning Of Il 23 Inhibitors Under Investigation Youtube
May 07, Interleukin23 (IL23) inhibitors are an important new class of drugs for the treatment of Crohn disease (CD) and ulcerative colitis (UC), both common causes of inflammation of the digestive tract Johnson &Background Ustekinumab is a monoclonal antibody targeting interleukin (IL)12 and IL23 and is approved for the treatment of plaque psoriasis, psoriatic arthritis, and Crohn's disease IL12 and IL23 have been implicated in systemic lupus erythematosusJohnson's Stelara (ustekinumab) is the only IL23 inhibitor currently approved to treat moderatetosevere CD and UC in the United States



Frontiers Why Inhibition Of Il 23 Lacked Efficacy In Ankylosing Spondylitis Immunology



Interleukin 17 And Interleukin 23 A Narrative Review Of Mechanisms Of Action In Psoriasis And Associated Comorbidities Springerlink
Introduction The monoclonal antibody targeting the shared p40 subunit of IL12 and IL23, namely ustekinumab, has been approved for Crohn's disease (CD) and has demonstrated promising results in the treatment of ulcerative colitis Several agents targeting the IL23specific p19 subunit are currently in various stages of developmentIL23 inhibitors have shown to be effective to both psoriasis and CD Case presentation Fortyone year old Chinese male patient who came to the hospital for psoriasis, developed severe gastrointestinal symptoms after using an IL17 inhibitor, and was diagnosed with Crohn'sMay 01, 19Objective Antitumour necrosis factor (TNF) antibodies are successfully used for treatment of Crohn's disease Nevertheless, approximately 40% of patients display failure to antiTNF therapy Here, we characterised molecular mechanisms that are associated with endoscopic resistance to antiTNF therapy Design Mucosal and blood cells were isolated from patients with Crohn's



Expansion Of Il 23 Receptor Bearing Tnfr2 T Cells Is Associated With Molecular Resistance To Anti Tnf Therapy In Crohn S Disease Gut



Stelara Ustekinumab Mechanism Of Action Plaque Psoriasis
Oct 30, 18Ustekinumab is a monoclonal antibody that binds to the p40 subunit common to both IL12 and IL23, thereby inhibiting receptor binding and suppressing both the IL12mediated Th1 pathway and the IL23mediated Th17 pathway 47 In contrast, the IL23specific inhibitors, such as guselkumab, bind to the p19 subunit of IL23, providing theOct 15, Overall, IL23 inhibitors have great potential in treating Crohn's disease In summary, we introduce a 41yearold male patient with psoriasis and CD who has aggravated CD after receiving IL17 inhibitors for psoriasis This patient has a 25year history of smoking, a risk factor for Crohn's diseaseMore than 35 million people globally live with inflammatory bowel diseases (IBD), including Crohn's disease, and the incidence continues to




Novel Pharmacological Approaches For Inflammatory Bowel Disease Targeting Key Intracellular Pathways And The Il 23 Il 17 Axis



Frontiers Role Of The Il23 Il17 Pathway In Crohn S Disease Immunology
Johnson's dual IL12/IL23 inhibitor Stelara (ustekinumab) for psoriasis, which rapidly achieved blockbuster status with addon indications in psoriatic arthritis and Crohn's and made $4bn in sales last yearThe interleukin (IL)23 axis is an emerging therapeutic target in IBD, including UC Initially approved for psoriasis, ustekinumab, an antibody blocking the p40 subunit of both IL12 and IL23, was approved for the treatment of Crohn's disease (CD) 9 and recently for UC asApr 12, 17Induction therapy with the selective interleukin23 inhibitor risankizumab in patients with moderatetosevere Crohn's disease a randomised, doubleblind, placebocontrolled phase 2 study Prof Brian G Feagan, MD William J Sandborn, MD Prof Geert D'Haens, PhD




Classes Of Biologics For The Treatment Of Ibd Alpco




Crohn S Disease And Ulcerative Colitis Show Unique Cytokine Profiles Abstract Europe Pmc



Clinical Trials Of Il 12 Il 23 Inhibitors In Inflammatory Bowel Disease Springerlink




Differential Roles For Interleukin 23 And Interleukin 17 In Intestinal Immunoregulation Sciencedirect




Deconvolution Of Monocyte Responses In Inflammatory Bowel Disease Reveals An Il 1 Cytokine Network That Regulates Il 23 In Genetic And Acquired Il 10 Resistance Gut




Il 23 In Inflammatory Bowel Diseases And Colon Cancer Sciencedirect




Stelara Ustekinumab For The Treatment Of Moderate To Severe Crohn S Disease Clinical Trials Arena




Historical Timeline Of Discoveries And Evolving Pathophysiologic Download Scientific Diagram




Paradoxical Gastrointestinal Effects Of Interleukin 17 Blockers Annals Of The Rheumatic Diseases




Pdf Induction Therapy With The Selective Interleukin 23 Inhibitor Risankizumab In Patients With Moderate To Severe Crohn S Disease A Randomised Double Blind Placebo Controlled Phase 2 Study Semantic Scholar




Interleukin Il 12 And Il 23 Structure And Receptors Notes The Il 12 Download Scientific Diagram




Why Did Il 23p19 Inhibition Fail In As A Tale Of Tissues Trials Or Translation Annals Of The Rheumatic Diseases
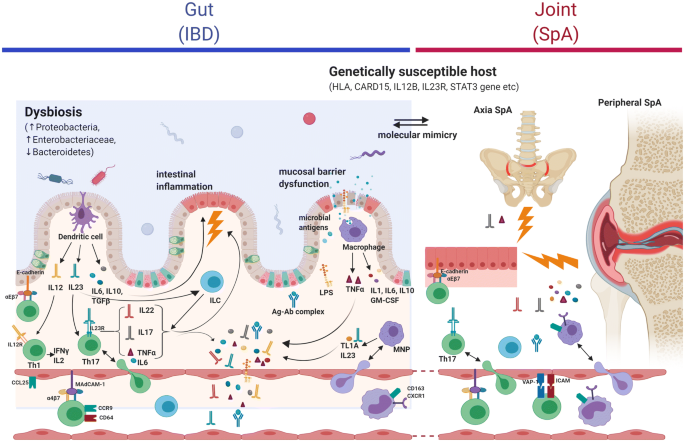



Defining The Phenotype Pathogenesis And Treatment Of Crohn S Disease Associated Spondyloarthritis Springerlink



Interleukin 23 In Ibd Pathogenesis Intechopen




Novel Pharmacological Approaches For Inflammatory Bowel Disease Targeting Key Intracellular Pathways And The Il 23 Il 17 Axis




Ustekinumab Binds To The P40 Subunit Of Il 12 And Il 23 Preventing Download Scientific Diagram




The Ibd Therapeutic Pipeline Is Primed To Produce Practical Gastro




Classes Of Biologics For The Treatment Of Ibd Alpco




All Are Equal Some Are More Equal Targeting Il 12 And 23 In Ibd Nda Itt




Efficacy And Safety Of Medi70 An Antibody Against Interleukin 23 In Patients With Moderate To Severe Crohn S Disease A Phase 2a Study Gastroenterology
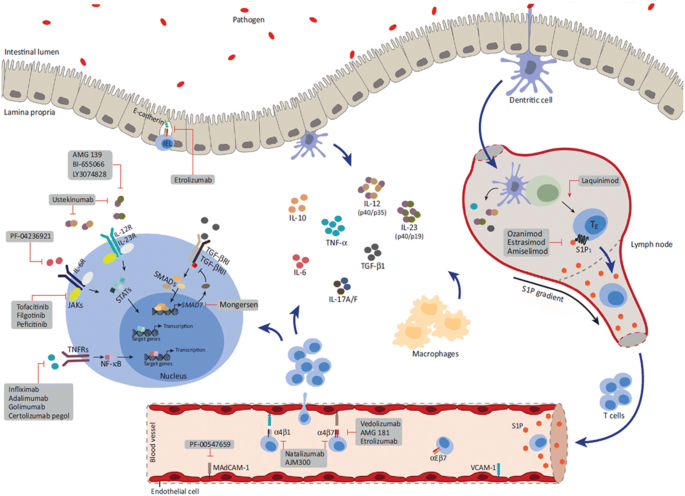



Emerging Therapies For Inflammatory Bowel Disease Springerlink




Crohn S Disease Market Will Experience Major Growth During Next Decade




Interleukin 23 Inhibition As A Strategy To Treat Immune Mediated Inflammatory Diseases European Medical Journal
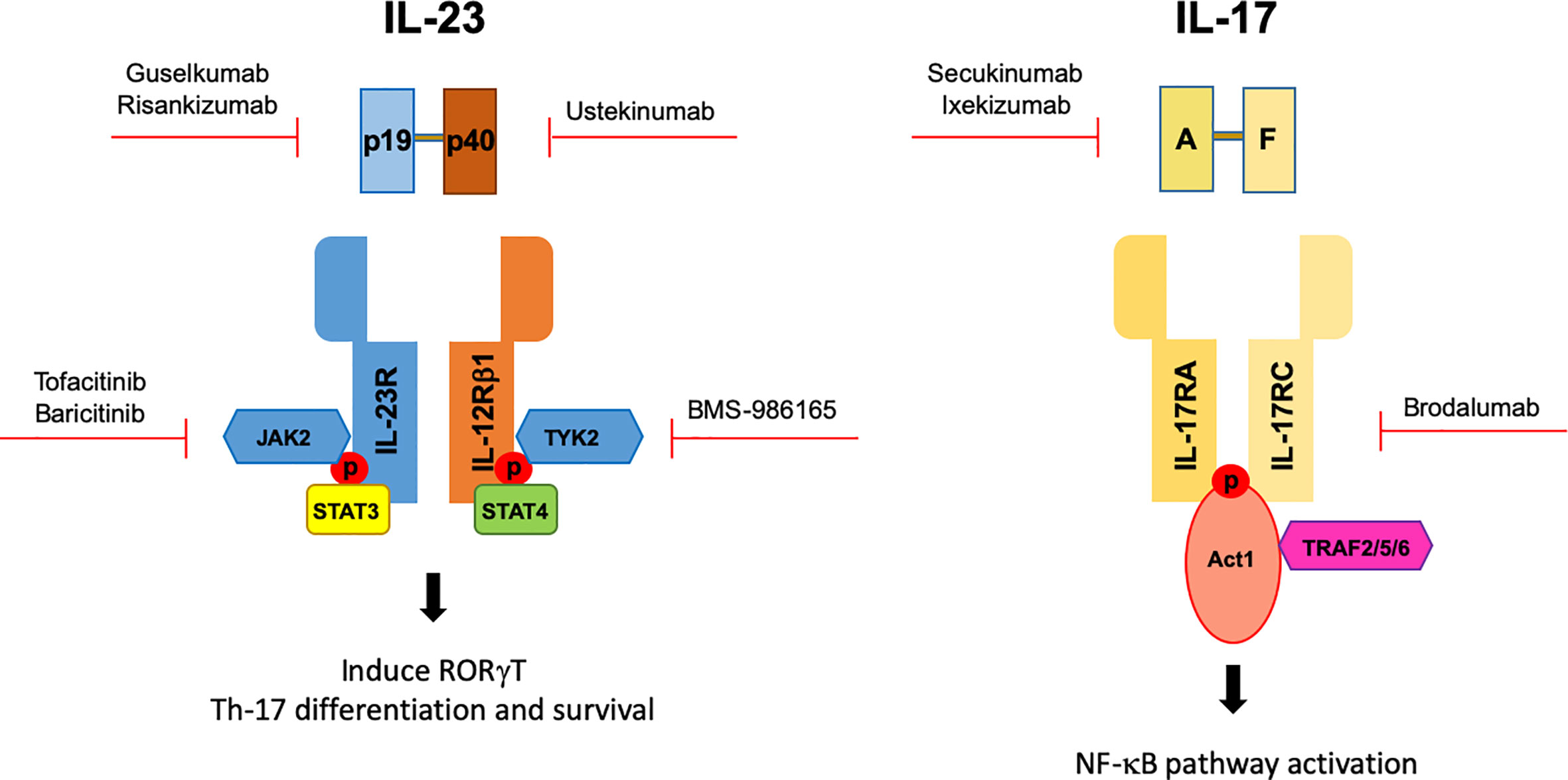



Frontiers The Il 17 Il 23 Axis And Its Genetic Contribution To Psoriatic Arthritis Immunology




Stat4 Activation By Leukemia Inhibitory Factor Confers A Therapeutic Effect On Intestinal Inflammation The Embo Journal




Il 12 Il 23 And Il 17 In Ibd Immunobiology And Therapeutic Targeting Semantic Scholar
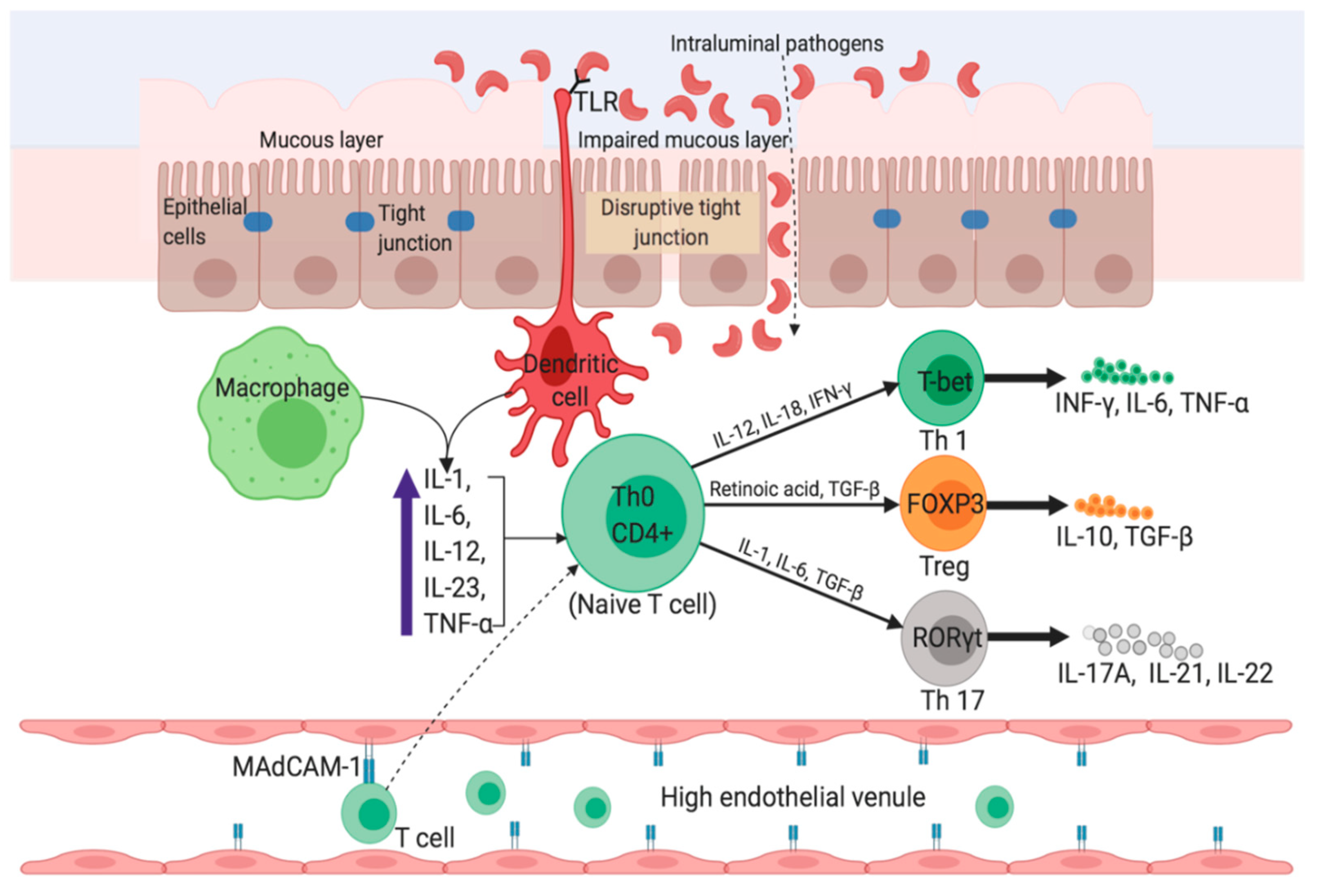



Jcm Free Full Text Revisiting Inflammatory Bowel Disease Pathology Treatments Challenges And Emerging Therapeutics Including Drug Leads From Natural Products Html




Can Il 23 Be A Good Target For Ulcerative Colitis Sciencedirect




Paradoxical Gastrointestinal Effects Of Interleukin 17 Blockers Annals Of The Rheumatic Diseases




Optimizing Management Of Ibd Beyond Tnf A Inhibitors
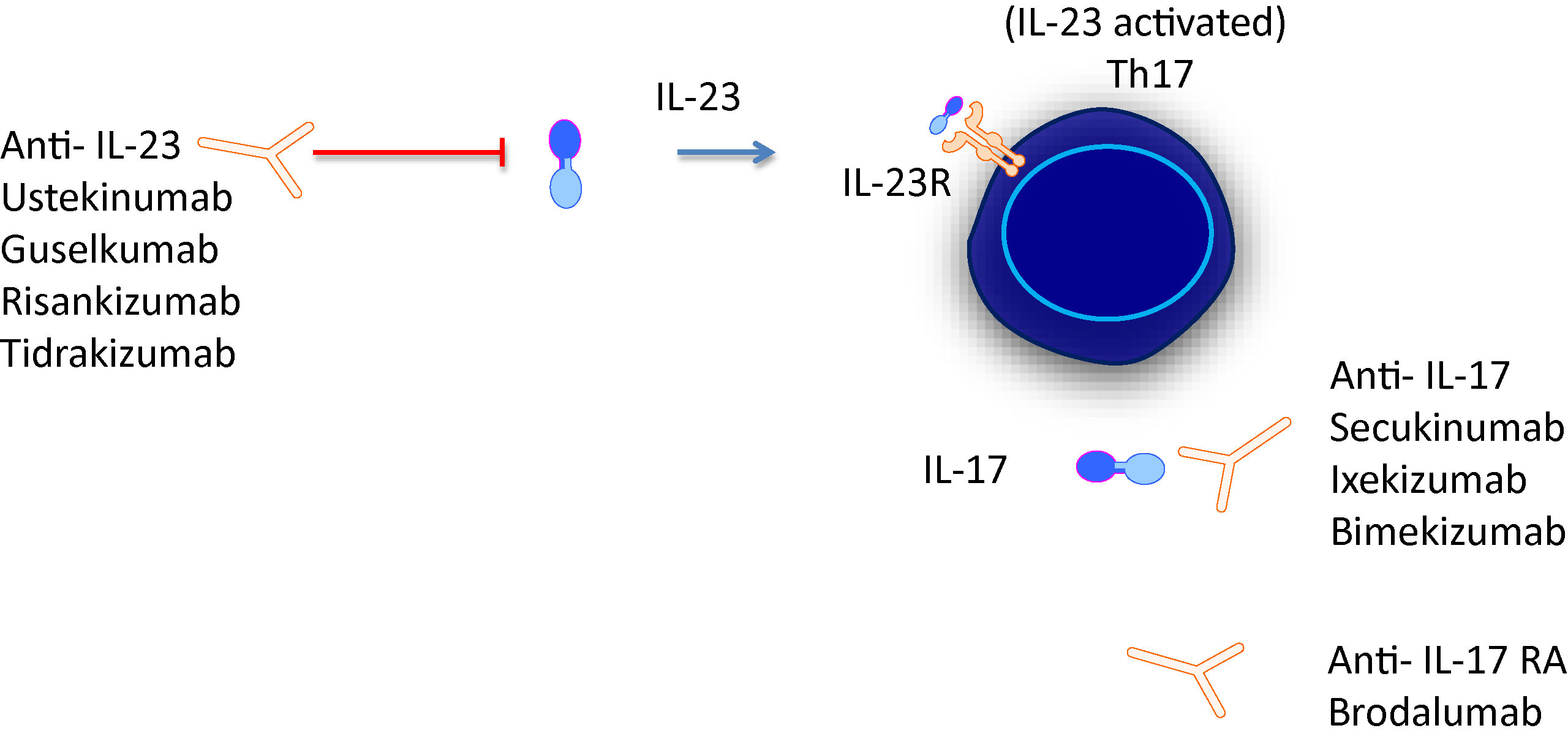



Frontiers Mini Review New Treatments In Psoriatic Arthritis Focus On The Il 23 17 Axis Pharmacology




Il 12 Il 23 And Il 17 In Ibd Immunobiology And Therapeutic Targeting Nature Reviews Gastroenterology Hepatology




Il 23 In Inflammatory Bowel Diseases And Colon Cancer Sciencedirect




Therapeutics Targeting The Il 23 And Il 17 Pathway In Psoriasis The Lancet




Ulcerative Colitis Today Tomorrow And The Future European Medical Journal
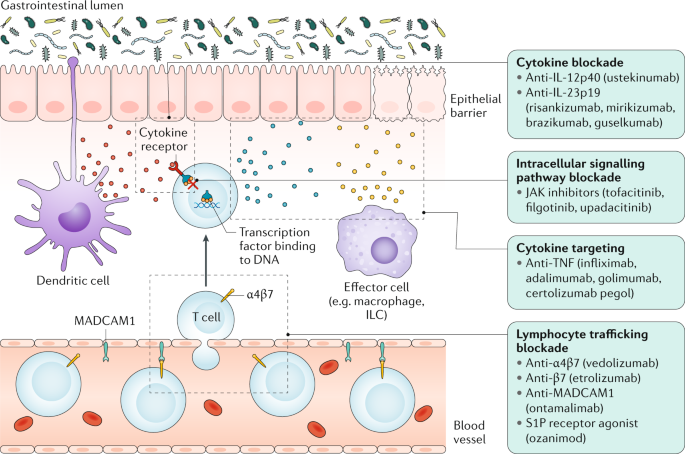



Interrogating Host Immunity To Predict Treatment Response In Inflammatory Bowel Disease Nature Reviews Gastroenterology Hepatology




Pdf Induction Therapy With The Selective Interleukin 23 Inhibitor Risankizumab In Patients With Moderate To Severe Crohn S Disease A Randomised Double Blind Placebo Controlled Phase 2 Study Semantic Scholar




Il 23 Producing Il 10ra Deficient Gut Macrophages Elicit An Il 22 Driven Proinflammatory Epithelial Cell Response Science Immunology




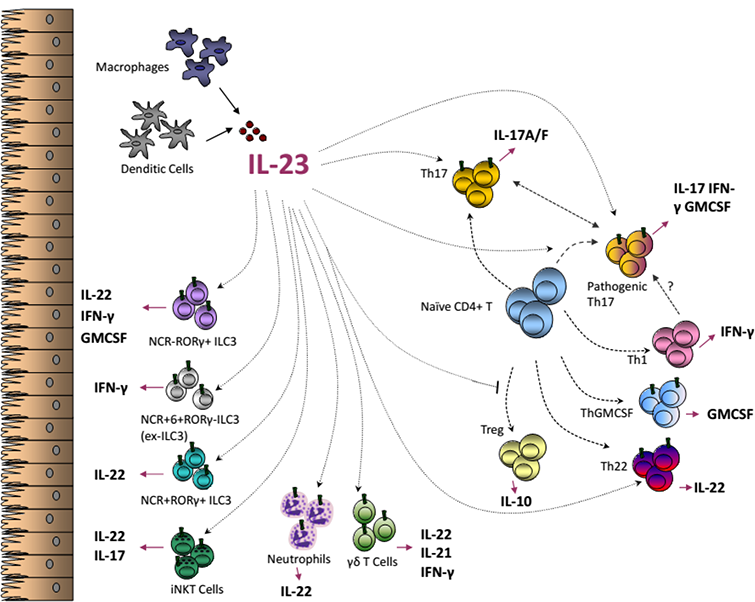
Interleukin 23 In Ibd Pathogenesis Intechopen




Jci Insight Therapeutic Manipulation Of Innate Lymphoid Cells




Discovery Of The Il 23 Il 17 Signaling Pathway And The Treatment Of Psoriasis The Journal Of Immunology




An Overview Of Novel And Emerging Therapies For Inflammatory Bowel Disease European Medical Journal




Classes Of Biologics For The Treatment Of Ibd Alpco




All Are Equal Some Are More Equal Targeting Il 12 And 23 In Ibd Nda Itt



Inflammatory Bowel Disease Insight Report Current Therapies Drug Pipeline And Outlook Biospace
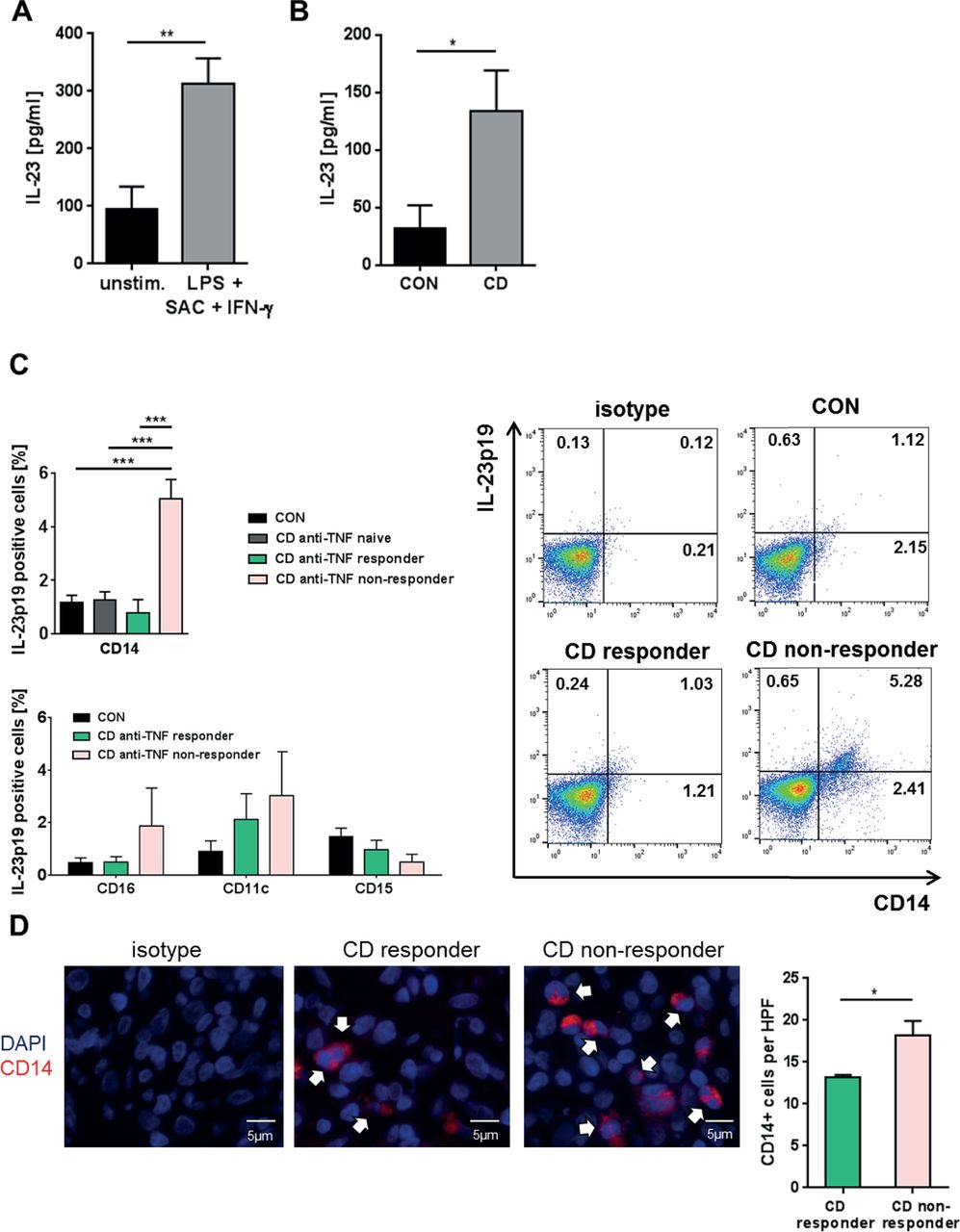



Expansion Of Il 23 Receptor Bearing Tnfr2 T Cells Is Associated With Molecular Resistance To Anti Tnf Therapy In Crohn S Disease Gut




The Proinflammatory Effect Of Prostaglandin E2 In Experimental Inflammatory Bowel Disease Is Mediated Through The Il 23 Il 17 Axis The Journal Of Immunology




Expansion Of Il 23 Receptor Bearing Tnfr2 T Cells Is Associated With Molecular Resistance To Anti Tnf Therapy In Crohn S Disease Gut



Biologics In Psoriasis The Next Generation Practical Dermatology




All Are Equal Some Are More Equal Targeting Il 12 And 23 In Ibd Nda Itt




Immunological Pathogenesis Of Inflammatory Bowel Disease
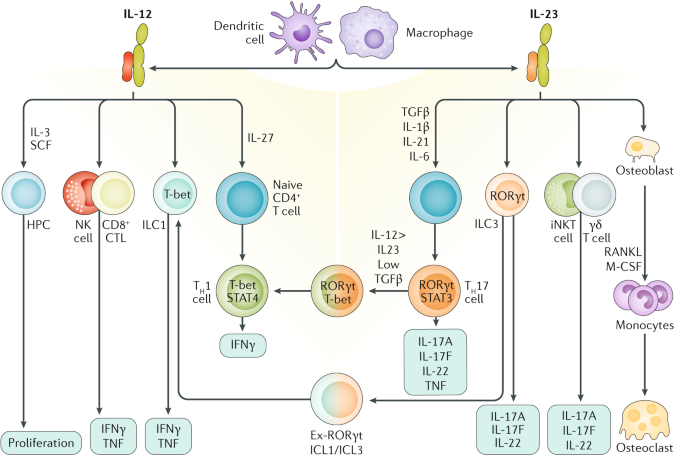



Il 12 Il 23 And Il 17 In Ibd Immunobiology And Therapeutic Targeting Nature Reviews Gastroenterology Hepatology
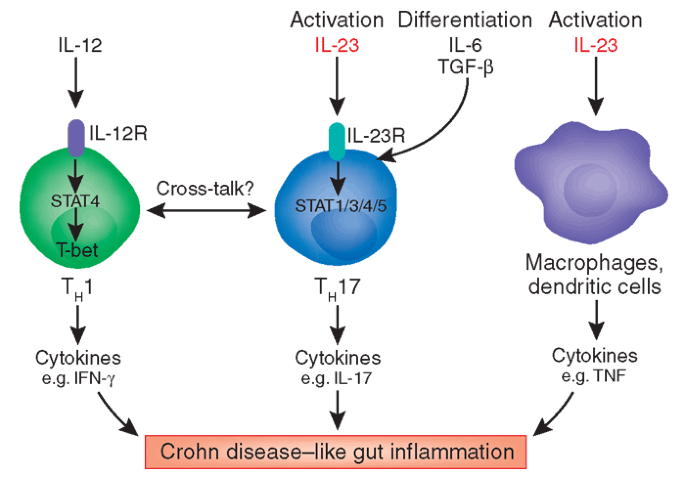



Il 23 A Master Regulator In Crohn Disease Nature Medicine
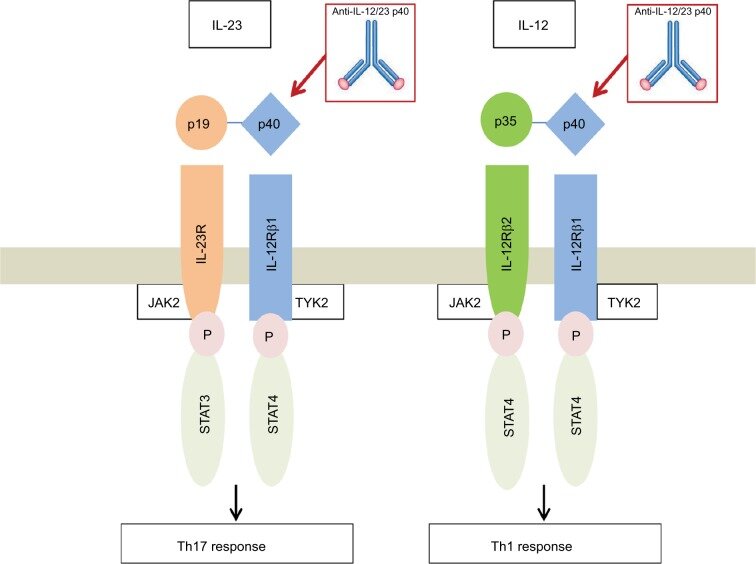



Schematic Representation Of Il 12 And Il 23 With Their Receptors And Download Scientific Diagram




Current Model Of The Pathophysiology Of Psoriasis Il 23 Bridges The Download Scientific Diagram
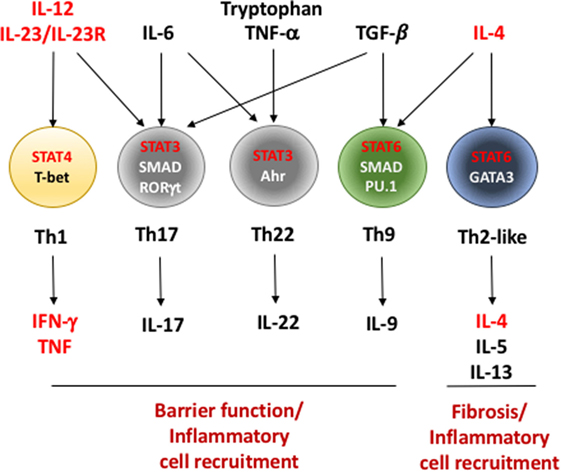



Frontiers Effector T Helper Cell Subsets In Inflammatory Bowel Diseases Immunology
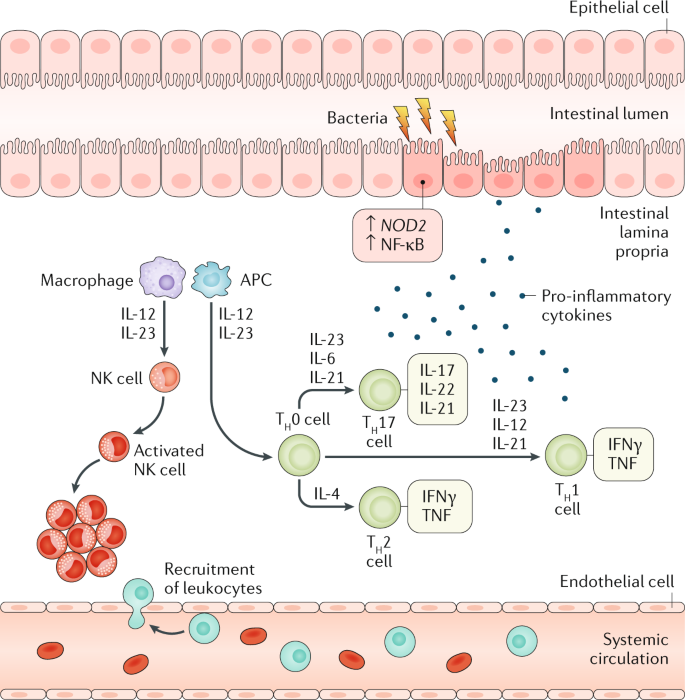



Crohn S Disease Nature Reviews Disease Primers




Optimizing Management Of Ibd Beyond Tnf A Inhibitors




Vitamin D Downregulates The Il 23 Receptor Pathway In Human Mucosal Group 3 Innate Lymphoid Cells Journal Of Allergy And Clinical Immunology




Emerging Treatments For Crohn S Disease Cells Surgery And Novel Therapeutics European Medical Journal



Latecomer Lilly Preps Its Il 23 Drug For Crohn S Disease Pmlive




Crohn S Disease Market Growth To 26 Fuelled By Interleukin Inhibitor And Anti Integrin Therapy Launches Globaldata
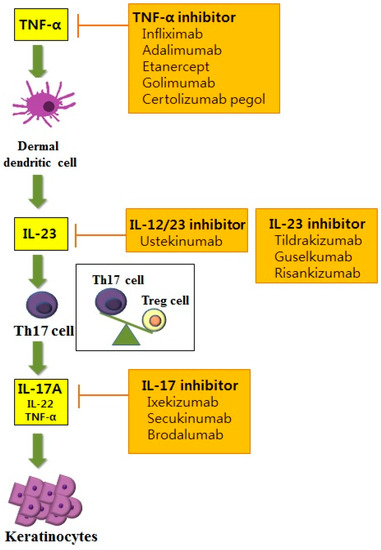



Ijms Free Full Text Molecular Mechanisms And Management Of A Cutaneous Inflammatory Disorder Psoriasis Html
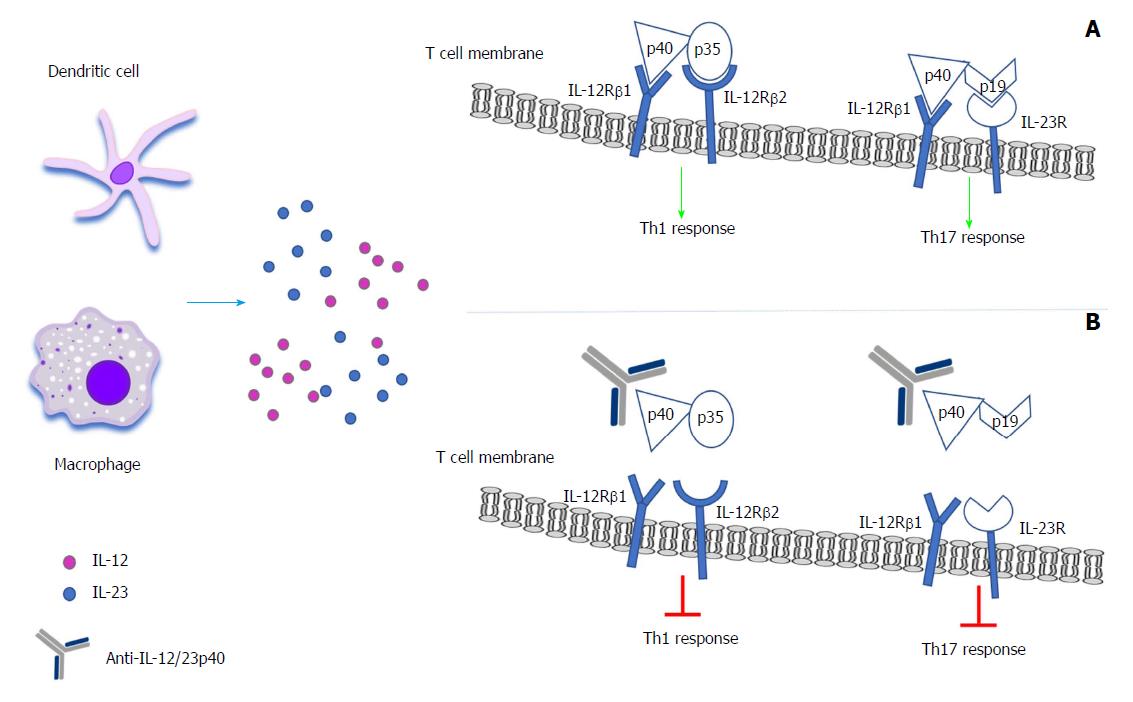



Interleukin 12 Interleukin 23 Pathway Biological Basis And Therapeutic Effect In Patients With Crohn S Disease




Immune Response In Inflammatory Bowel Disease Ibd Tlr Toll Like Download Scientific Diagram
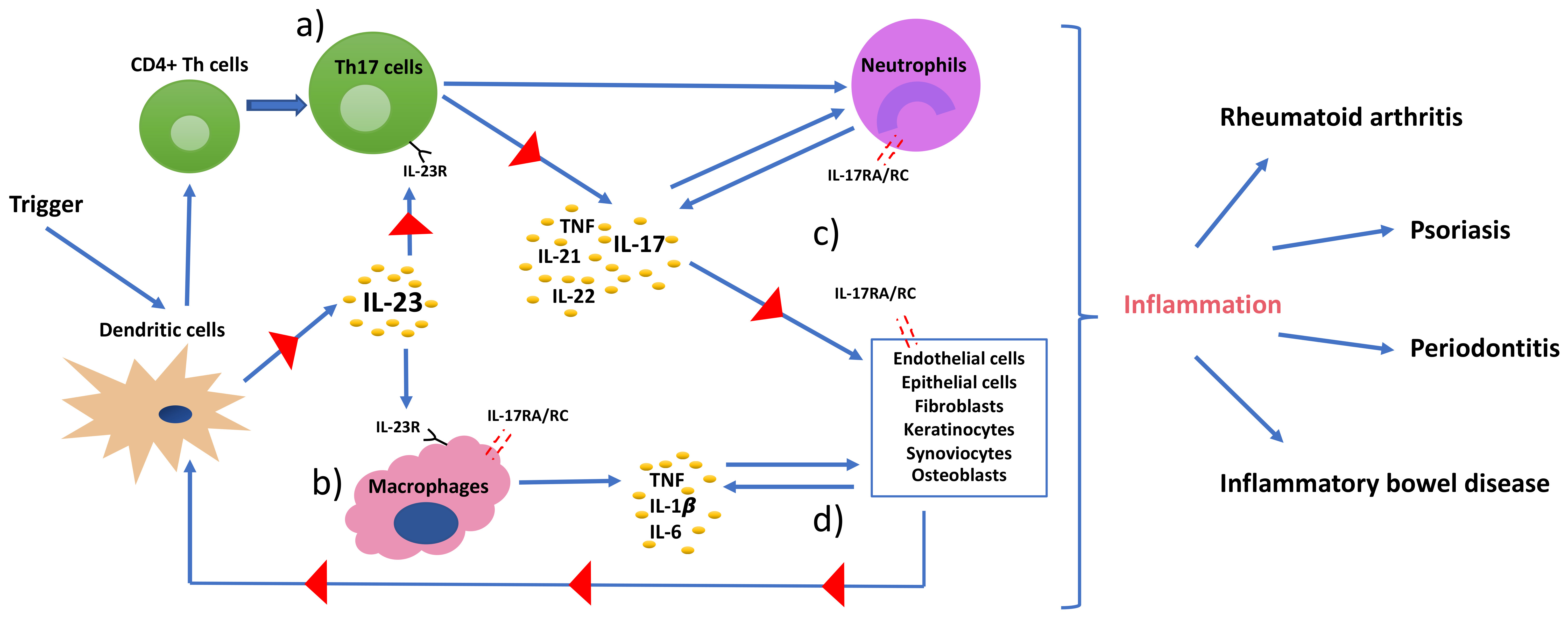



Ijms Free Full Text Th17 Cells And The Il 23 Il 17 Axis In The Pathogenesis Of Periodontitis And Immune Mediated Inflammatory Diseases Html



0 件のコメント:
コメントを投稿